Contact distribution partner
Für Wasser, Industrielle Prozesse, Verkehr & Umwelt kontaktieren Sie unseren Hauptsitz.
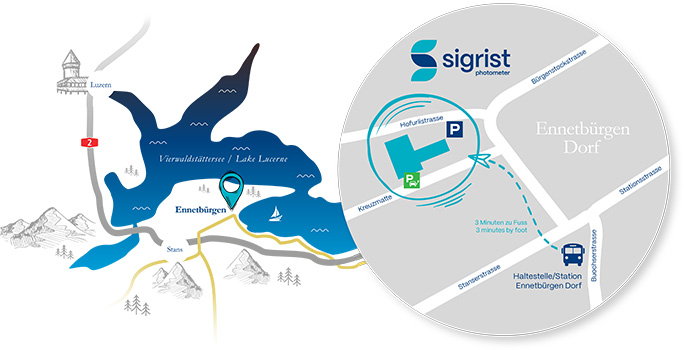
For water, industrial processes, traffic & environment please contact our headquarters
Für Wasser, Industrielle Prozesse, Verkehr & Umwelt kontaktieren Sie unseren Hauptsitz.
For water, industrial processes, traffic & environment please contact our headquarters